The effectiveness of MEPSEVII was determined using several tests, including:

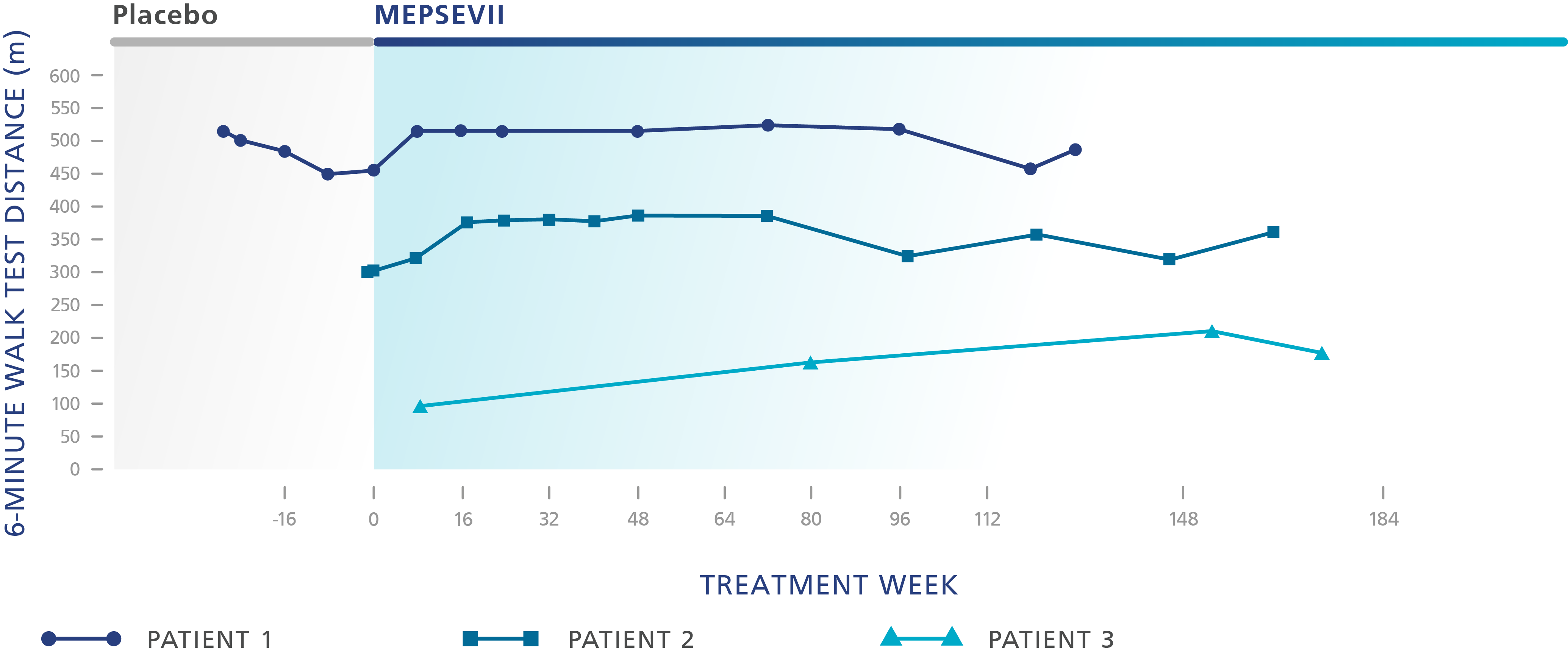
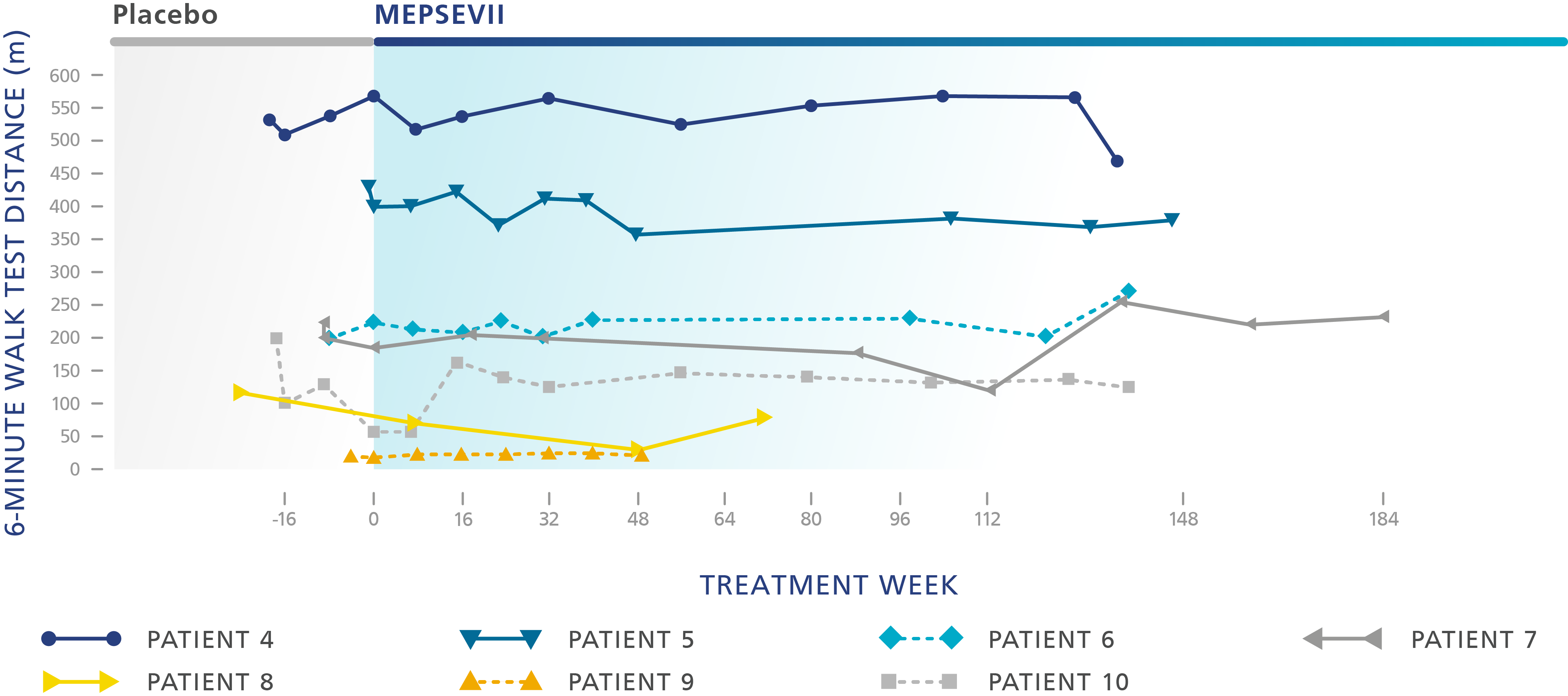
Six-minute walk test (6MWT)
which measures how far someone can walk in 6 minutes. It is an effective way of measuring how well the different systems in the body work together
Breathing ability
which is measured to see how healthy the respiratory system is

Liver and spleen size
which measures whether these organs are of normal or abnormal size
Due to the nature of MPS VII and the differences in the patients in this trial, you should talk with your doctor about what you may expect from treatment with MEPSEVII
In clinical trials, some patients who took MEPSEVII experienced improvement or maintained walking distance, improvement in breathing ability, and reduction in organ size
- A severe allergic reaction called anaphylaxis has occurred with MEPSEVII treatment, as early as the first dose.
- Your doctor will monitor you closely for symptoms of an allergic reaction while you are receiving MEPSEVII and for 60 minutes after your injection.
- Your doctor will immediately discontinue the MEPSEVII infusion if you experience anaphylaxis.
- Your doctor should talk to you about the signs and symptoms of anaphylaxis and about getting medical treatment if you have symptoms after leaving the doctor’s office or treatment center.
- The most common side effects of MEPSEVII are:
- Leakage of MEPSEVII into the surrounding tissue during infusion
- Diarrhea
- Rash
- Severe allergic reaction (anaphylaxis)
- Infusion site swelling
- Swelling around the infusion site
- Severe itching of the skin
- One patient experienced a seizure during a fever while taking MEPSEVII.
- are pregnant, think you may be pregnant, or plan to become pregnant. There is not enough experience to know if MEPSEVII may harm your unborn baby.
- are breastfeeding or plan to breastfeed. There is not enough experience to know if MEPSEVII passes into your breast milk. Talk with your doctor about the best way to feed your baby while you receive MEPSEVII.
These are not all the possible side effects of MEPSEVII. Call your doctor for medical advice about side effects.