INDICATION
MEPSEVII is a recombinant human lysosomal beta glucuronidase indicated
in pediatric and adult patients
for the treatment of
Mucopolysaccharidosis VII (MPS VII, Sly
syndrome).
Limitations of Use
The effect of MEPSEVII on the central nervous system manifestations of
MPS VII has not been determined.
Boxed warning and additional Important Safety Information
WARNING: ANAPHYLAXIS
-
Anaphylaxis has occurred with MEPSEVII administration, as early as the first dose,
therefore
appropriate medical support should be readily available when MEPSEVII is
administered.
-
Closely observe patients during and for 60 minutes after MEPSEVII infusion.
-
Immediately discontinue the MEPSEVII infusion if the patient experiences
anaphylaxis.
-
Anaphylaxis to MEPSEVII was reported in 2 of 20 patients in the
clinical program. The two patients
with anaphylaxis to MEPSEVII during the clinical trials had one occurrence each and
tolerated
subsequent infusions of MEPSEVII, without recurrence.
-
Consider the risks and benefits of re-administering MEPSEVII
following anaphylaxis.
-
Manifestations included respiratory distress, cyanosis, decreased
oxygen saturation, and hypotension.
-
Prior to discharge, inform patients of the signs and symptoms of
anaphylaxis and instruct them to
seek immediate medical care if symptoms occur.
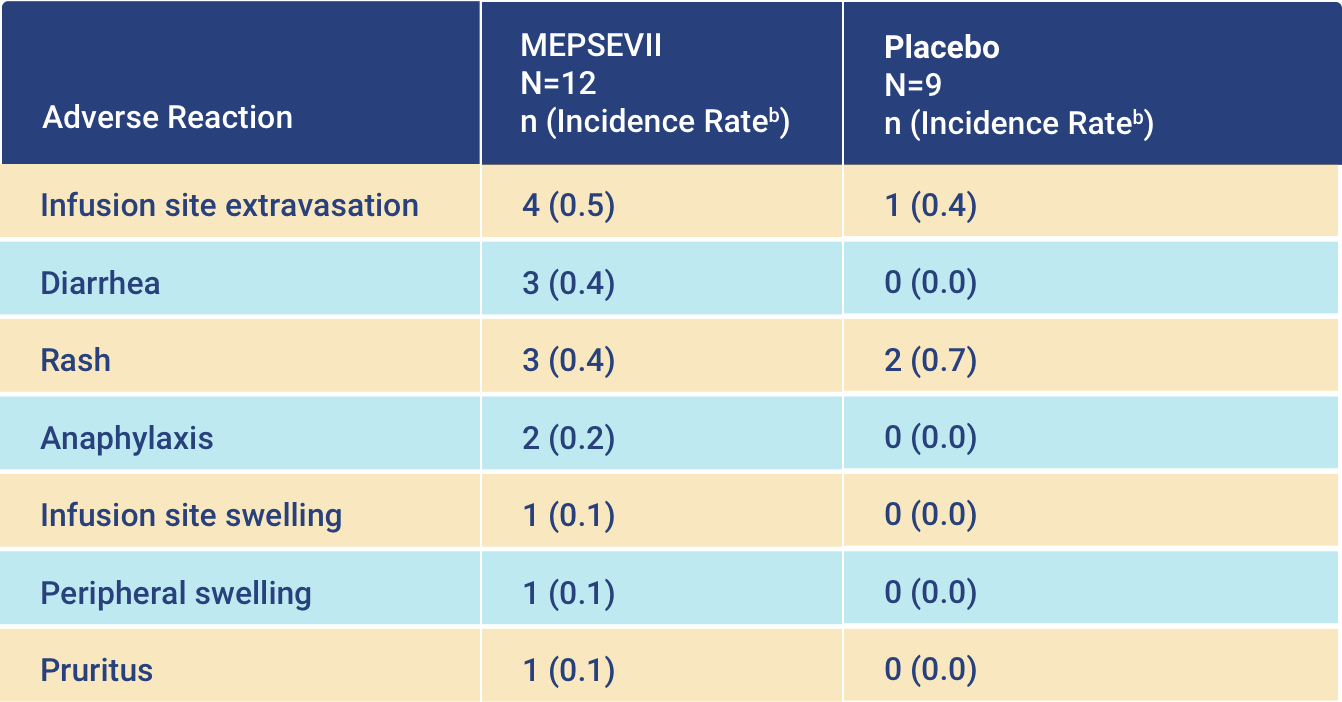
Adverse Reactions
-
In a clinical trial, the most common adverse reactions occurring
with MEPSEVII treatment included
infusion site extravasation, diarrhea, rash, anaphylaxis, infusion site swelling, peripheral
swelling, and pruritus.
-
One patient experienced a febrile convulsion during MEPSEVII
treatment. The patient subsequently
was re-challenged without recurrence and continued on treatment.
Use in Specific Populations
-
There are no available data on MEPSEVII use in pregnant women to
determine a drug-associated risk
of adverse developmental outcomes.
-
There are no data on the presence of MEPSEVII in either human or
animal milk, the effects on the
breastfed infant, or the effects on milk production. The developmental and health benefits
of
breastfeeding should be considered along with the mother’s clinical need for MEPSEVII and
any
potential adverse effects on the breastfed infant from MEPSEVII or from the underlying
maternal
condition.
You may report side effects to the FDA at (800) FDA-1088
or www.fda.gov/medwatch.
You may also report side effects to Ultragenyx at 1-888-756-8657.
Please see full Prescribing
Information,
including the BOXED WARNING, for a complete
discussion of the risks associated with MEPSEVII.